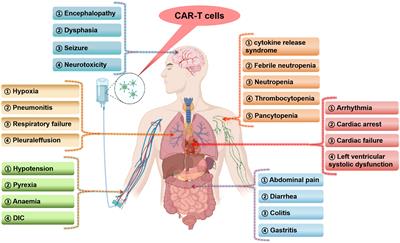
Serious adverse events and coping strategies of CAR-T cells in the treatment of malignant tumors
Source : https://www.frontiersin.org/articles/10.3389/fimmu.2022.1079181/full
Chimeric antigen receptor T (CAR-T) cells technology has been successfully used in the treatment of B cell-derived hematological tumors and multiple myeloma. CAR-T cells are also being studied in a...
Conclusions: In this review, we summarized the serious adverse events of CAR-T cells in the treatment of malignant tumors by reading literature and searching relevant clinical studies, and discussed the management and treatment of serious adverse events in an effort to provide theoretical support for clinicians who deal with such patients.

The Burden of a Multiple Myeloma Diagnosis on Patients and Caregivers | CEOR
Multiple myeloma (MM) is a malignant clonal plasma cell proliferative disorder characterized by uncontrolled and progressive increase of monoclonal paraprotein leading to specific end-organ damage. The proliferating multiple myeloma cells...
Conclusions: NDMM is burdensome for patients and caregivers in the first year after diagnosis. TNE patients are more dependent on caregivers and incur higher care costs than TE patients. Despite the financial, physical, and emotional burden, HRQoL remains stable possibly indicating resilience and illness adjustment amongst patients and...

Recent Advances in the Use of Chimeric Antigen Receptor-Expressing T-Cell Therapies for Treatment of Multiple Myeloma - PubMed
Source : https://pubmed.ncbi.nlm.nih.gov/36411210/
Data collected to date support CAR-T therapies holding substantial promise for patients with heavily pretreated RRMM relative to other currently available therapies. Additional real-world data will help provide further insights...
Conclusions: Data collected to date support CAR-T therapies holding substantial promise for patients with heavily pretreated RRMM relative to other currently available therapies. Additional real-world data will help provide further insights into the comparative efficacy and safety profiles of these treatments in RRMM as these treatments become...

Innovation in BCMA CAR-T therapy: Building beyond the Model T - PubMed
Source : https://pubmed.ncbi.nlm.nih.gov/36505779/
Autologous chimeric antigen receptor T-cell (CAR-T) therapies targeting B-cell maturation antigen (BCMA) have revolutionized the field of multiple myeloma in the same way that the Ford Model T revolutionized the...
Conclusions: Conclusions/Relevance: Just as the Model T set a benchmark for car manufacturing over 100 years ago, idecabtagene vicleucel and ciltacabtagene autoleucel have now set the starting point for BCMA CAR-T therapy with their approvals. As with any emerging technology, whether automotive or cellular, the best in innovation and...

LocoMMotion: a prospective, non-interventional, multinational study of real-life current standards of care in patients with relapsed and/or refractory multiple myeloma - PubMed
Source : https://pubmed.ncbi.nlm.nih.gov/35332278/
Despite treatment advances, patients with multiple myeloma (MM) often progress through standard drug classes including proteasome inhibitors (PIs), immunomodulatory drugs (IMiDs), and anti-CD38 monoclonal antibodies (mAbs). LocoMMotion (ClinicalTrials.gov identifier: NCT04035226)...
Relevance: Despite treatment advances, patients with multiple myeloma (MM) often progress through standard drug classes including proteasome inhibitors (PIs), immunomodulatory drugs (IMiDs), and anti-CD38 monoclonal antibodies (mAbs). LocoMMotion is the first prospective study of real-life standard of care (SOC) in triple-class exposed (received...

