Molecular and clinical features of papillary thyroid cancer in adult patients with a non-classical phenotype
Source : https://www.frontiersin.org/articles/10.3389/fendo.2023.1138100/full
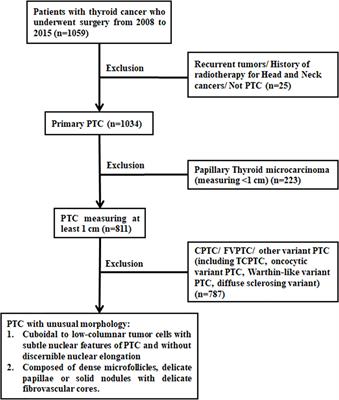
PurposeGenotyping is fundamental in papillary thyroid cancer (PTC) and helps to enhance diagnosis and prognosis and determine appropriate treatments. The phenotype-genotype association in PTC was previously studied, with BRAF V600E...
Conclusions: Our study retrospectively screened a large cohort of different thyroid tissue samples, and presented the histopathological and genetic features of a non-classical phenotype of PTC from 24 patients. It may contribute to diagnose in PTC, and patients of these non-classical phenotype may benefit from targeted therapy, compared...

Agnostic Approvals in Oncology: Getting the Right Drug to the Right Patient with the Right Genomics - PubMed
Source : https://pubmed.ncbi.nlm.nih.gov/37111371/
The .gov means it's official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you're on a federal government site. The site is secure....
Conclusions: Precision medicine is constantly evolving, and with the improvement of diagnostic tools that allow a wider genomic definition of the tumor, tissue-agnostic targeted therapies are a promising treatment strategy tailored to the specific tumor genomic profile, leading to improved survival outcomes.

Selpercatinib Controls Central Nervous System Disease in Patients With NSCLC With RET Fusion - PubMed
Source : https://pubmed.ncbi.nlm.nih.gov/37087123/
Selpercatinib Controls Central Nervous System Disease in Patients With NSCLC With RET Fusion
Relevance: In clinical aspects, early initiation of selpercatinib is associated with decreased rates of CNS metastasis formation and progression; therefore, selpercatinib may play a preventive role.
-
 Advances in RET Therapy2yrKey Points • Source: Journal of Thoracic Oncology • Conclusions/Relevance: “With this report by Murciano-Goroff et al., selpercatinib has likely joined osimertinib and lorlatinib as agents with activity in the CNS for their respective Show More
Advances in RET Therapy2yrKey Points • Source: Journal of Thoracic Oncology • Conclusions/Relevance: “With this report by Murciano-Goroff et al., selpercatinib has likely joined osimertinib and lorlatinib as agents with activity in the CNS for their respective Show More

Cabozantinib in the treatment of locally advanced DTC | CMAR
The incidence of thyroid cancers has significantly increased over the last several decades, in part due to the frequent use of thyroid ultrasound diagnosing more cases of this disease. Despite...
Conclusions/Relevance: Molecular testing for driver mutations or gene fusions, such as BRAF V600E or RET or NTRK fusions, has become standard recommendations for RAIR DTC patients due to excellent treatment options with highly selective targeted therapies, most RAIR DTC patients do not harbor such aberrations or...

Re-Evaluating Real-World Evidence in RET Fusion-Positive NSCLC: Are Randomized Clinical Trials Needed? - PubMed
Source : https://pubmed.ncbi.nlm.nih.gov/37087114/
The .gov means it's official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you're on a federal government site. The site is secure....
Summary: RET-MAP provides RWE on treatment outcomes for patients with RET+ lung cancer. This study offers a lens into the natural history of this disease across different stages of cancer and various treatment types. Although phase 3 studies comparing frontline RET TKI to standard of care are ongoing (NCT04222972, NCT04819100), FDA approved...
