Medullary Thyroid Cancer- Updates and Challenges
Source : https://academic.oup.com/edrv/advance-article/doi/10.1210/endrev/bnad013/7173174?login=false
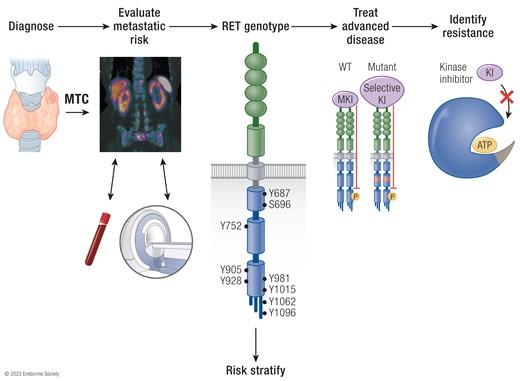
Abstract. A personalised approach to the management of Medullary Thyroid Cancer (MTC) presents several challenges, however in the past decade significant progre
Conclusions/Relevance: Here we discuss changes in paradigms for MTC patients; from determining RET alteration status upfront to novel techniques for the evaluation of this heterogenous disease. Successes and challenges with kinase inhibitor use will illustrate how managing this rare malignancy continues to evolve.

GENIE BPC NSCLC cohort: a real-world repository integrating standardized clinical and genomic data for 1,846 patients with non-small cell lung cancer
Abstract. Purpose: We describe the clinical and genomic landscape of the non-small cell lung cancer (NSCLC) cohort of the AACR Project GENIE Biopharma Collaborative (BPC). Experimental Design: 1,846 patients with...
Conclusions: The GENIE BPC cohort provides comprehensive clinico-genomic data for patients with NSCLC, which can improve understanding of real-world patient outcomes.

FDA Approval Summary: Selpercatinib for the treatment of advanced RET fusion-positive solid tumors
Abstract. On September 21, 2022, the U.S. Food and Drug Administration (FDA) granted accelerated approval to selpercatinib (Retevmo®, Eli Lilly and Company) for the treatment of adult patients with locally...
Conclusions/Relevance: On September 21, 2022, the U.S. Food and Drug Administration (FDA) granted accelerated approval to selpercatinib (Retevmo®, Eli Lilly and Company) for the treatment of adult patients with locally advanced or metastatic solid tumors with a rearranged during transfection (RET) gene fusion that have progressed on or...

Gene Fusion Detection in NSCLC Routine Clinical Practice: Targeted-NGS or FISH? - PubMed
Source : https://pubmed.ncbi.nlm.nih.gov/37190044/
The ability to identify the broadest range of targetable gene fusions is crucial to facilitate personalized therapy selection for advanced lung adenocarcinoma (LuADs) patients harboring targetable receptor tyrosine kinase (RTK)...
Conclusion: The targeted RNA NGS analysis of LuADs allows accurate RTK fusion detection; nevertheless, standard methods such as FISH should not be dismissed, as they can crucially contribute to the completion of the molecular characterization of LuADs and, most importantly, the identification of patients as candidates for targeted therapies.

Gene Fusion Detection in NSCLC Routine Clinical Practice: Targeted-NGS or FISH? - PubMed
Source : https://pubmed.ncbi.nlm.nih.gov/37190044/
The ability to identify the broadest range of targetable gene fusions is crucial to facilitate personalized therapy selection for advanced lung adenocarcinoma (LuADs) patients harboring targetable receptor tyrosine kinase (RTK)...
Conclusion: The targeted RNA NGS analysis of LuADs allows accurate RTK fusion detection; nevertheless, standard methods such as FISH should not be dismissed, as they can crucially contribute to the completion of the molecular characterization of LuADs and, most importantly, the identification of patients as candidates for targeted therapies.

