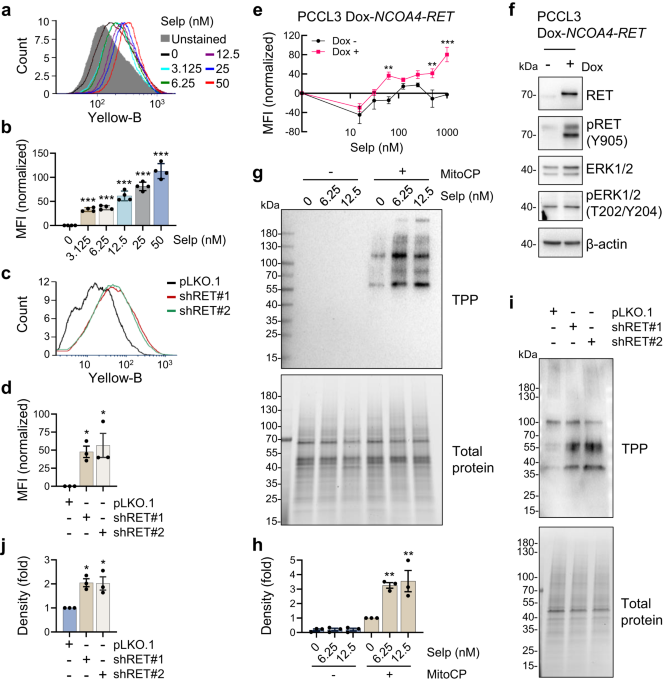
Selpercatinib Combination With the Mitochondria-Targeted Antioxidant MitoQ Effectively Suppresses RET-Mutant Thyroid Cancer
Source : https://www.nature.com/articles/s41698-024-00536-7
Genetic alternation of REarranged during Transfection (RET) that leads to constitutive RET activation is a crucial etiological factor for thyroid cancer. RET is known to regulate mitochondrial processes, although the...
The 2 patients in this report did not tolerate standard doses of selpercatinib due to adverse effects, but they did tolerate reduced doses of selpercatinib when combined with mitochondria-targeted ubiquinone.

Impact of the Quality of Resected Thyroid Cancer Tissue Sample on Next-Generation Sequencing Testing
Source : https://pubmed.ncbi.nlm.nih.gov/38226479/
Activating rearranged during transfection (RET) proto-oncogene alterations can be identified using next-generation sequencing (NGS) of tumor DNA/RNA. We assessed factors associated with NGS (Oncomine Dx Target Test [ODxTT]) success for...
Quality assessment of nucleic acid extracted from archive tissue samples is important for achieving high next-generation sequencing success rates in clinical practice.

Comprehensive Clinicopathological, Molecular, and Methylation Analysis of Mesenchymal Tumors With NTRK and Other Kinase Gene Aberrations
Source : https://pubmed.ncbi.nlm.nih.gov/38332737/
Alterations in kinase genes such as NTRK1/2/3, RET, and BRAF underlie infantile fibrosarcoma (IFS), the emerging entity 'NTRK-rearranged spindle cell neoplasms' included in the latest WHO classification, and a growing...
Novel PWWP2A::RET, NUMA1::RET, ITSN1::RAF1, and CAPZA2::MET fusions, which the authors reported in mesenchymal tumors for the first time, were detected by RNA sequencing.

Exposure-Response Relationships for Pralsetinib in Patients With RET-Altered Thyroid Cancer or RET Fusion-Positive Nonsmall Cell Lung Cancer
Source : https://pubmed.ncbi.nlm.nih.gov/38337106/
Pralsetinib is a highly potent oral kinase inhibitor of oncogenic RET (rearranged during transfection) fusions and mutations. Pralsetinib received approval from the United States Food and Drug Administration for the...
In patients with RET fusion-positive NSCLC, this analysis found that patients who started on 400 mg QD had greatly improved PFS compared with patients who started on ≤300 mg QD.

LIBRETTO-431: Is it Time to Reconsider Randomized Phase 3 Trials for Uncommon Oncogenic Drivers in Non-Small-Cell Lung Cancer?
Source : https://pubmed.ncbi.nlm.nih.gov/38340705/
The recently published results of LIBRETTO-431 1 pave the way for a new standard of care in the first-line setting for RET-fusion-positive NSCLCs, which raises important clinical questions not only...
In this viewpoint, the authors raised important clinical questions about the therapeutic landscape of advanced NSCLC and the drug development process in the era of uncommon molecular subtypes.

