Home > Focus Areas > Leukemia and Lymphoma Connect > Post

Case report: Zanubrutinib-induced dermatological toxicities: A single-center experience and review
Source : https://www.frontiersin.org/articles/10.3389/fonc.2022.941633/full
Zanubrutinib, a next-generation non-covalent Bruton's tyrosine kinase (BTK) inhibitor, shows great efficacy in the treatment of B cell malignancies. Some patients may experience a series of side effects after the treatment of zanubrutinib. Grade 4 dermatological toxicities are rare, which present as severe rash and skin infection.
Conclusion: The majority of patients can be relieved with symptomatic treatment, but a very small percentage of patients may face discontinuation of the drug.

• Source: Frontiers in Oncology
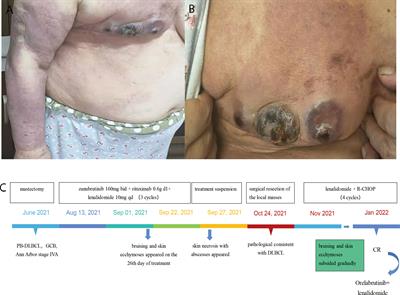
• Relevance: “Here, we retrospectively reported three cases of dermatological toxicities induced by zanubrutinib. All three patients developed grade 4 skin adverse effects, which lead to a suspension of treatment in two patients.”
• These cases are the first to be reported in China. The authors want to raise clinical awareness about zanubrutinib-induced dermatological toxicities and the importance of drug withdrawal.
• Some experts suggest that direct binding to BTK and other ‘off-target’ kinases results in adverse events related to BTK inhibition. Zanubrutinib inhibits BTK very selectively and minimizes off-target inhibition of other kinases.
• “The most likely mechanism of zanubrutinib-induced skin rash appears to be off-target inhibition of EGFR. Inhibition of c-kit and platelet-derived growth factor receptors is another mechanism proposed for zanubrutinib-induced drug eruption …. these rash types, particularly those that appeared within the first month of treatment, could be related to the transient hyperlymphocytosis associated with BTK, which is caused by CLL cells egressing from lymph nodes and spleen,” the authors wrote.
• In clinical trials, 10% or more of patients taking zanubrutinib experienced skin reactions
• The authors recommend symptomatic treatment, including moisturizers and topical steroids, as well as antibiotics in case of skin infection.