Ibrutinib-associated dermatologic toxicities: A systematic review and meta-analysis
Source : https://www.sciencedirect.com/science/article/abs/pii/S1040842822001202?via=ihub
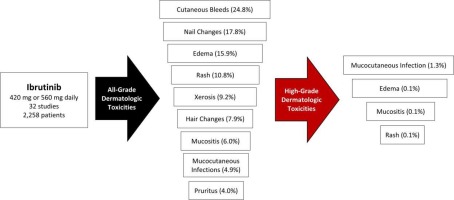
Ibrutinib-associated dermatologic toxicities are common * Toxicities included cutaneous bleeds, mucocutaneous infections, and nail changes * High-grade toxicities included mucocutaneous infections, rash, mucositis, and edema * Toxicity identification and management are needed to improve patient outcomes The scope of dermatologic adverse events to ibrutinib has not been systematically described.
Relevance: It is imperative that clinicians familiarize themselves with ibrutinib-associated dermatologic toxicities to learn how to manage them, prevent discontinuation, and improve patient outcomes.

• Source: Critical Reviews in Oncology/Hematology
• Conclusion: “It is imperative that clinicians familiarize themselves with ibrutinib-associated dermatologic toxicities to learn how to manage them, prevent discontinuation, and improve patient outcomes.”
• This systematic review spanned 32 clinical studies with 2258 patients. The trials examined ibrutinib monotherapy for cancer or chronic graft-versus-host disease.
• Derm toxicity secondary to ibrutinib is common and includes cutaneous bleed, mucocutaneous infections, and nail changes.
• High-grade toxicities include mucocutaneous infections, mucositis, rash, and edema.