
Pirtobrutinib Targets BTK C481S in Ibrutinib-Resistant CLL but Second-Site BTK Mutations Lead to Resistance
Research Article | Aishath Naeem, Filippo Utro, Qing Wang, Justin Cha, Mauno Vihinen, Stephen P. Martindale, Yinglu Zhou, Yue Ren, Svitlana Tyekucheva, Annette S. Kim, Stacey M. Fernandes, Gordon Saksena,...
Relevance: We employed longitudinal whole exome sequencing on two patients whose disease progressed on pirtobrutinib and identified selection of alternative-site BTK mutations, providing clinical evidence that secondary BTK mutations lead to resistance to non-covalent BTKi.

Resistance to Bruton tyrosine kinase inhibition in chronic lymphocytic leukaemia and non-Hodgkin lymphoma - PubMed
Source : https://pubmed.ncbi.nlm.nih.gov/36029036/
Bruton tyrosine kinase inhibitors (BTKi) have transformed the therapeutic landscape of chronic lymphocytic leukaemia (CLL) and non-Hodgkin lymphoma. However, primary and acquired resistance to BTKi can be seen due to...
Relevance: We summarise ongoing clinical investigations aimed at overcoming resistance via use of BTKi-containing combined therapies or the novel non-covalent BTKi. The review article targets an audience of clinical practitioners, clinical investigators and translational researchers.

MCL-133 Pirtobrutinib, a Highly Selective, Non-Covalent (Reversible) BTK Inhibitor in Previously Treated Mantle Cell Lymphoma: Updated Results From the Phase 1/2 BRUIN Study - PubMed
Source : https://pubmed.ncbi.nlm.nih.gov/36164120/
Pirtobrutinib demonstrated promising efficacy in heavily pretreated, poor-prognosis MCL following multiple prior lines of therapy, including a covalent BTKi. Pirtobrutinib was well tolerated and exhibited a wide therapeutic index. Updated...
Conclusions: Pirtobrutinib demonstrated promising efficacy in heavily pretreated, poor-prognosis MCL following multiple prior lines of therapy, including a covalent BTKi. Pirtobrutinib was well tolerated and exhibited a wide therapeutic index. Updated data, including approximately 60 new patients with MCL and an additional 10 months since the...

MCL-135 BRUIN MCL-321, a Phase 3 Open-Label, Randomized Study of Pirtobrutinib Versus Investigator Choice of BTK Inhibitor in Patients With Previously Treated, BTK Inhibitor Naïve Mantle Cell Lymphoma (Trial in Progress) - PubMed
Source : https://pubmed.ncbi.nlm.nih.gov/36164121/
1 Eli Lilly and Company, Indianapolis, USA. 2 Oxford University Hospitals NHS Foundation Trust, Churchill Cancer Center, Oxford, United Kingdom. 3 Medical College of Wisconsin, Milwaukee, USA. 4 Service d'hématologie...
Objective: Determine whether pirtobrutinib is superior to investigator's choice of covalent BTKi in patients with previously treated, BTKi-naïve MCL. Design: BRUIN MCL-321 is a randomized, open-label, global phase 3 study comparing pirtobrutinib monotherapy versus investigator's choice of covalent BTKi monotherapy (ibrutinib, acalabrutinib, or...
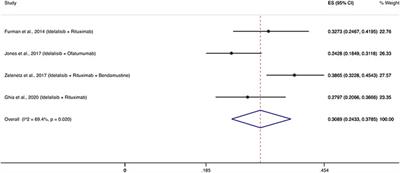
Targeted therapies in CLL/SLL and the cumulative incidence of infection: A systematic review and meta-analysis
Source : https://www.frontiersin.org/articles/10.3389/fphar.2022.989830/full
Background: Patients with chronic lymphocytic leukemia (CLL)/small lymphocytic lymphoma (SLL) are prone to infections.Aims: Provide a pooled estimate of the cumulative incidence for infections that fulfilled the criteria associated with...
Conclusion: Patients with CLL/SLL who receive targeted therapies may develop severe infections at significant rates that, in addition to disease stage and other complications, depend on the mechanism of action of the used drug. Surveillance for infections and development of effective prophylactic strategies are critical for patients with...

