
Pirtobrutinib in Covalent BTK-Inhibitor Pre-treated Mantle Cell Lymphoma - PubMed
Source : https://pubmed.ncbi.nlm.nih.gov/37192437/
Pirtobrutinib is a first-in-class novel non-covalent (reversible) BTKi, and the first BTKi of any kind to demonstrate durable efficacy following prior cBTKi therapy in heavily pre-treated relapsed/refractory MCL. Pirtobrutinib was...
Conclusion: Pirtobrutinib is a first-in-class novel non-covalent (reversible) BTKi, and the first BTKi of any kind to demonstrate durable efficacy following prior cBTKi therapy in heavily pre-treated relapsed/refractory MCL. Pirtobrutinib was well tolerated with low rates of treatment discontinuation due to toxicity.
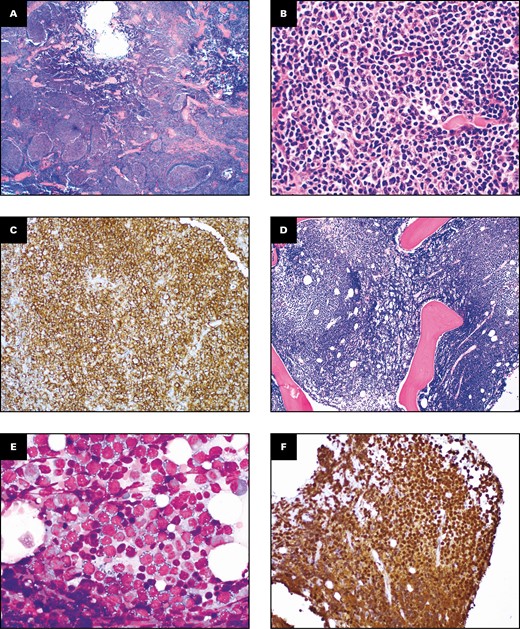
Progression of follicular lymphoma and related entities: Report from the 2021 SH/EAHP Workshop
The morphologic, immunophenotypic, and molecular features of transformations from indolent B-cell lymphomas were reviewed using a case-based approach. Transformations of follicular lymphomas to aggressive lymphomas with acquisition of an additional...
Conclusions: Together, these unique cases and their accompanying molecular and cytogenetic data suggest potential mechanisms for and unusual patterns of transformation in B-cell lymphomas and indicate numerous opportunities for further study.

Validation of MYC and BCL6 rapid break apart digital fluorescence in situ hybridization assays for clinical use
Source : https://www.ncbi.nlm.nih.gov/pmc/articles/PMC10165167/
Michael Liew, 1 Leslie Rowe, 1 Kristina Moore, 1 Emily Aston, 1 Kathryn O'Brien, 1 Maria Longhurst, 1 Jason Kenney, 1 Marshall Priest, 1 Wenhua Zhou, 1 Diane Wilcock, 1...
Conclusions: This study validates the use of the SureFISH MYC and BCL6 probes on formalin fixed paraffin embedded (FFPE) tissue sections using a hybridization time of 1.5 hours that shortened the overall workflow by 14.5 hours. The process described also provides a standardized framework for validating digital FISH assays in the future.

Unmet clinical needs in the use of zanubrutinib in malignant lymphomas (Waldenström macroglobulinemia, marginal zone lymphoma and mantle cell lymphoma): A consensus-based position paper from an ad hoc expert panel - PubMed
Source : https://pubmed.ncbi.nlm.nih.gov/37165730/
Zanubrutinib has been approved for the treatment of patients with different lymphoproliferative disorders, and now represents a major breakthrough in the treatment of patients resistant or relapsing after the recommended...
Relevance: This article presents the results of group discussion among an ad hoc constituted panel of experts aimed at identifying and addressing unmet clinical needs (UCNs) in the use of zanubrutinib in the lymphomas which have received the approval of use, specifically Waldenström macroglubulinemia, marginal zone lymphoma and mantle cell...

Pirtobrutinib: A New Hope for patients with BTK-inhibitor refractory lymphoproliferative disorders
Review Article | Philip A Thompson, Constantine Tam; Pirtobrutinib: A New Hope for patients with BTK-inhibitor refractory lymphoproliferative disorders. Blood 2023; blood.2023020240. doi: https://doi.org/10.1182/blood.2023020240 Download citation file: You do not...
Conclusions/Relevance: We summarize existing pre-clinical and clinical data with pirtobrutinib.

