
Pharmacodynamic Biomarkers Evidentiary Considerations for Biosimilar Development and Approval - PubMed
Source : https://pubmed.ncbi.nlm.nih.gov/36178447/
A biosimilar is a biological product that is highly similar to and has no clinically meaningful differences from a U.S. Food and Drug Administration (FDA)-approved reference product. The development and...
Conclusion/Relevance: FDA’s applied regulatory science activities related to PD biomarkers for biosimilars conducted in support of the FDA’s Biosimilars Action Plan are reviewed. This included conducting three clinical studies to address information gaps about PD biomakers for biosimilars and inform general methodological best practices. In...

The Role of PD Biomarkers in Biosimilar Development - To Get the Right Answer One Must First Ask the Right Question - PubMed
Source : https://pubmed.ncbi.nlm.nih.gov/36148870/
The potential for pharmacodynamic (PD) biomarkers to improve the efficiency of biosimilar product development and regulatory approval formed the premise for the virtual workshop Pharmacodynamic Biomarkers for Biosimilar Development and...
Conclusion/Relevance: We must carefully consider the core requirements and timelines inherent in biosimilar development and how they occur in parallel rather than in the series we see for originator products. In order to improve the efficiency of biosimilar development, we need to ask the right questions based on a full understanding of how...

Rapid infusion of infliximab biosimilars and the incidence and severity of infusion-related reactions in patients with inflammatory bowel disease - PubMed
Source : https://pubmed.ncbi.nlm.nih.gov/36134561/
Rapid infusions of infliximab biosimilars were not associated with an increase in the incidence of infusion reactions compared with: rapid infusion of infliximab reference product, standard infusion of infliximab biosimilars,...
Conclusions: Rapid infusions of infliximab biosimilars were not associated with an increase in the incidence of infusion reactions compared with: rapid infusion of infliximab reference product, standard infusion of infliximab biosimilars, or standard infusion of infliximab reference product. This should reassure clinicians that rapid infusions...
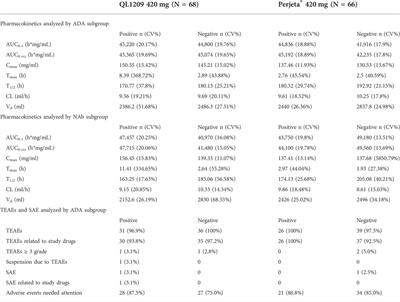
A randomized, double-blind, parallel control study to evaluate the biosimilarity of QL1209 with Perjeta® in healthy male subjects
Source : https://www.frontiersin.org/articles/10.3389/fphar.2022.953641/full
Purpose: This is the first study to compare the pharmacokinetics, safety and, immunogenicity of QL1209, a biosimilar of Perjeta®.Methods: This study was a randomized, double-blind, parallel-controlled clinical trial evaluating the...
Conclusion: The results of this comparative clinical pharmacology study demonstrated the PK similarity of QL1209 (420 mg: 14 ml) and Perjeta® (420 mg: 14 ml) and there was no significant difference in safety and immunogenicity between QL1209 and Perjeta® manufactured by Roche Pharma AG.
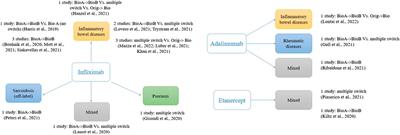
Switching Among Biosimilars: A Review of Clinical Evidence
Source : https://www.frontiersin.org/articles/10.3389/fphar.2022.917814/full
Biological medicines have improved patients' outcomes, but their high costs may limit access. Biosimilars, alternatives that have demonstrated high similarity in terms of quality, safety, and efficacy to an already...
Conclusion: Switching studies seem difficult to perform and unnecessary with the body of evidence suggesting no real problems in practice coupled with stringent regulatory requirements. Monitoring the use of biosimilars in clinical practice could support clinical decision-making, rational use of biological medicines, and help to further realize...

