
Management of Adverse Reactions for BCMA-Directed Therapy in Relapsed Multiple Myeloma: A Focused Review
Source : https://www.mdpi.com/2077-0383/12/17/5539
Anti-B-cell maturation antigen therapies consisting of bispecific antibodies, antibody-drug conjugates, and chimeric antigen receptor T cells have shown promising results in relapsed refractory multiple myeloma (RRMM). However, the severe side...
Conclusions: MM is the second most common hematologic malignancy, which remains incurable despite advances in its treatment secondary to drug resistance and the presence of minimal residual disease. Recently, BCMA agents have shown favorable outcomes in heavily pre-treated RRMM cohorts, as evidenced by multiple published clinical trials....

Post-infusion Costs Associated with Idecabtagene Vicleucel Treatment for Patients with Relapsed/Refractory Multiple Myeloma in the KarMMa Trial - Advances in Therapy
Source : https://link.springer.com/article/10.1007/s12325-023-02623-w
Introduction Patients with triple-class exposed relapsed/refractory multiple myeloma (RRMM) have poor outcomes with substantial healthcare costs. Idecabtagene vicleucel (ide-cel), a B-cell maturation antigen (BCMA)-directed chimeric antigen receptor (CAR) T cell...
Conclusions: Extrapolation of costs based on HCRU data from patients receiving single-infusion of ide-cel in the KarMMa trial showed substantially reduced HCRU and costs over 2 years after initial treatment. Most costs were incurred during the first month after ide-cel infusion, likely attributable to the 14-day inpatient stay required by the...
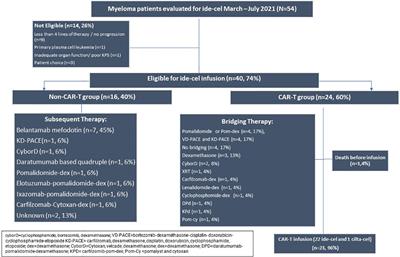
"Waitlist mortality" is high for myeloma patients with limited access to BCMA therapy
Source : https://www.frontiersin.org/journals/oncology/articles/10.3389/fonc.2023.1206715/full
BackgroundThe first-in-class approved BCMA CAR-T therapy was idecabtagene vicleucel (ide-cel), approved in March 2021, for RRMM patients who progressed after 4 or more lines of therapy. Despite the promising outcomes,...
Conclusion(s): Many patients who were eligible for ide-cel were not able to secure a timely slot in 2021. Mortality was higher in this group, due to a lack of comparable alternatives. Increasing alternate options as well as improvement in manufacturing and access is an area of high importance to improve RRMM outcomes.

Outcome of carfilzomib/pomalidomide-based regimens after daratumumab-based treatment in relapsed multiple myeloma: A Canadian Myeloma Research Group Database analysis - PubMed
Source : https://pubmed.ncbi.nlm.nih.gov/37574220/
Our observations demonstrate the poor outcome of MM patients when standard regimens based on carfilzomib and/or pomalidomide are utilized directly after daratumumab-based therapy given in the relapsed setting. Novel therapies,...
Conclusion: Our observations demonstrate the poor outcome of MM patients when standard regimens based on carfilzomib and/or pomalidomide are utilized directly after daratumumab-based therapy given in the relapsed setting. Novel therapies, including immune therapies, are urgently needed to improve the outcomes of these daratumumab-exposed...

Single-agent belantamab mafodotin in patients with relapsed/refractory multiple myeloma: Final analysis of the DREAMM-2 trial - PubMed
Source : https://pubmed.ncbi.nlm.nih.gov/37622738/
This final analysis of DREAMM-2 confirms that in patients with triple-class refractory RRMM, single-agent belamaf results in durable and clinically meaningful responses with a manageable safety profile.
Conclusions: This final analysis of DREAMM-2 confirms that in patients with triple-class refractory RRMM, single-agent belamaf results in durable and clinically meaningful responses with a manageable safety profile.

