The Prevalence of MYD88 L265P and TNFAIP3 Mutations and Their Correlations with Clinico-Hematological Profile in Egyptian Patients with Diffuse Large B Cell Lymphoma
Source : https://journal.waocp.org/article_90728.html
Background: Activated B-cell-like (ABC) subtype of diffuse large B-cell lymphoma (DLBCL) is characterized by chronic active B-cell receptor signaling and a constitutive activation of the NF-KB pathway. MYD88 L265P mutation...
Conclusion: MYD88 L265P and to lesser extent TNFAIP3 mutations are major mutations in ABC- DLBCL and may be predictive factors for poor OS in ABC- DLBCL patients.
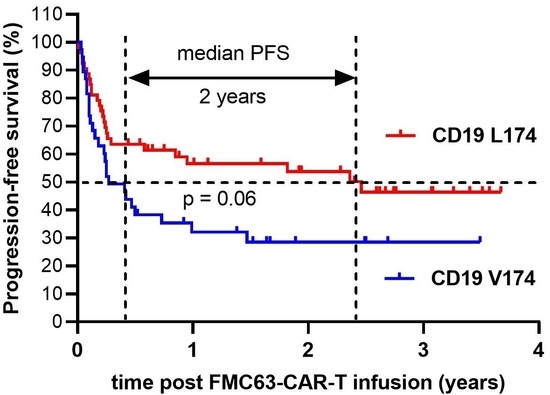
Clinical Impact of Single Nucleotide Polymorphism in CD-19 on Treatment Outcome in FMC63-CAR-T Cell Therapy
Source : https://www.mdpi.com/2072-6694/15/11/3058
Chimeric antigen receptor (CAR)-T cell therapy is effective in patients with relapsed or refractory diffuse large B-cell lymphoma (r/r DLBCL) with response rates of 63-84% and complete response observed in...
Conclusions: In this retrospective observational study, we investigated the treatment outcome related to the single nucleotide polymorphism rs2904880 encoding leucine or valine at amino acid position 174 (L174V) on the CD19 antigen in a DLBCL patient cohort receiving FMC63-anti-CD19-CAR-T therapy. Our data suggest that the single nucleotide...

Is it a chimera? A systematic review of the economic evaluations of CAR-T cell therapy - an update - PubMed
Source : https://pubmed.ncbi.nlm.nih.gov/37288738/
The updated results corroborated the previously reported favorable cost-effectiveness ratio of CAR-T. They also pointed out differences among CAR-T agents. However, their budget impact emerges as a significant barrier in...
Expert Opinion: The updated results corroborated the previously reported favorable cost–effectiveness ratio of CAR-T. They also pointed out differences among CAR-T agents. However, their budget impact emerges as a significant barrier in the reimbursement process. Any proposed Managed Entry Agreement must integrate the ingrained uncertainty...

Value for Money of CAR-T Cell Therapy for Patients with Diffuse Large B-cell Lymphoma in China: Evidence from a Cost-Effectiveness Analysis - Applied Health Economics and Health Policy
Source : https://link.springer.com/article/10.1007/s40258-023-00817-5
Objective This research assesses the cost effectiveness of Axicabtagene ciloleucel (Axi-cel), Tisagenlecleucel (Tis-cel), Relmacabtagene autoleucel (Rel-cel) and Lisocabtagene maraleucel (Lis-cel) against standard of care (SOC) for patients with diffuse large...
Conclusion: Our results demonstrated that CAR-T treatments are not cost effective in any-line settings for DLBCL patients at the WHO-recommended willingness-to-pay threshold (CNY257,241 per QALY) in the base-case analysis. Price reduction of CAR-T therapies is the main approach for lowering ICERs and ensuring that the drug costs are...

Value for Money of CAR-T Cell Therapy for Patients with Diffuse Large B-cell Lymphoma in China: Evidence from a Cost-Effectiveness Analysis - Applied Health Economics and Health Policy
Source : https://link.springer.com/article/10.1007/s40258-023-00817-5
Objective This research assesses the cost effectiveness of Axicabtagene ciloleucel (Axi-cel), Tisagenlecleucel (Tis-cel), Relmacabtagene autoleucel (Rel-cel) and Lisocabtagene maraleucel (Lis-cel) against standard of care (SOC) for patients with diffuse large...
Conclusion: Our results demonstrated that CAR-T treatments are not cost effective in any-line settings for DLBCL patients at the WHO-recommended willingness-to-pay threshold (CNY257,241 per QALY) in the base-case analysis. Price reduction of CAR-T therapies is the main approach for lowering ICERs and ensuring that the drug costs are...

