
Less necessity of adjuvant S-1 treatment in non-monarchE-eligible patients - PubMed
Source : https://pubmed.ncbi.nlm.nih.gov/37162105/
MonarchE criteria accurately identify patients who are prone to relapse. Moreover, although POTENT criteria also suggested a reasonable capacity for recurrence prediction, there was no significant difference in recurrence between...
Conclusion: MonarchE criteria accurately identify patients who are prone to relapse. Moreover, although POTENT criteria also suggested a reasonable capacity for recurrence prediction, there was no significant difference in recurrence between POTENT non-eligible patients and the patients who were POTENT but not monarchE eligible. This might...

Response to Pralsetinib in Multi-Drug-Resistant Breast Cancer With CCDC6-RET Mutation - PubMed
Source : https://pubmed.ncbi.nlm.nih.gov/37141396/
Triple-negative breast cancers (TNBC) represent a pathological subtype of breast cancer, which are characterized by strong invasiveness, high metastasis rate, low survival rate, and poor prognosis, especially in patients who...
Conclusions/Relevance: This case report is believed to be the first demonstrating clinical efficacy of pralsetinib in a patient with multidrug-resistant breast cancer with a coiled-coil domain-containing protein 6-rearranged during transfection mutation.
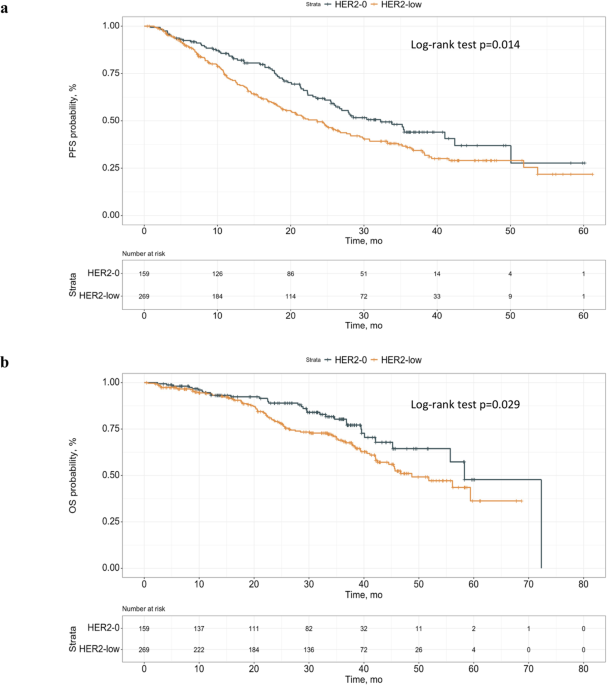
Prognostic significance of HER2-low status in HR-positive/HER2-negative advanced breast cancer treated with CDK4/6 inhibitors - npj Breast Cancer
Source : https://www.nature.com/articles/s41523-023-00534-1
Whether Human Epidermal growth factor Receptor 2 (HER2)-low status has prognostic significance in HR + /HER2- advanced Breast Cancer (aBC) patients treated with first-line Endocrine Therapy plus CDK 4/6 inhibitors remains unclear....
Abstract: Whether Human Epidermal growth factor Receptor 2 (HER2)-low status has prognostic significance in HR + /HER2- advanced Breast Cancer (aBC) patients treated with first-line Endocrine Therapy plus CDK 4/6 inhibitors remains unclear. In 428 patients evaluated, HER2-low status was independently associated with significantly worse...

A Gene Panel Associated With Abemaciclib Utility in ESR1-Mutated Breast Cancer After Prior Cyclin-Dependent Kinase 4/6-Inhibitor Progression - PubMed
Source : https://pubmed.ncbi.nlm.nih.gov/37141550/
For ESR1-MUT MBC with resistance to ET and palbociclib, PFS on abemaciclib is longer for patients with CDKi-R(-) than CDKi-R(+). Although a small and retrospective data set, this is the...
Conclusion: For ESR1-MUT MBC with resistance to ET and palbociclib, PFS on abemaciclib is longer for patients with CDKi-R(–) than CDKi-R(+). Although a small and retrospective data set, this is the first demonstration of a genomic panel associated with abemaciclib sensitivity in the postpalbociclib setting. Future directions include testing...

US Food and Drug Administration Expanded Adjuvant Indication of Abemaciclib in High-Risk Early Breast Cancer - PubMed
Source : https://pubmed.ncbi.nlm.nih.gov/37104738/
The .gov means it's official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you're on a federal government site. The site is secure....
Relevance: On March 3, 2023, the US Food and Drug Administration (FDA) expanded the approval of abemaciclib, in combination with standard endocrine therapy (ET), for high-risk patients on the basis of clinicopathologic features alone removing the requirement of a Ki-67 score ≥20%.

